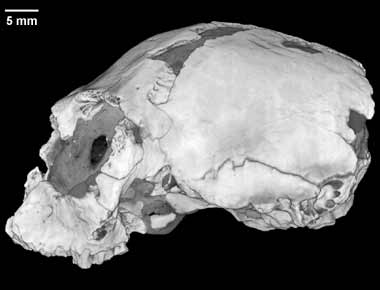
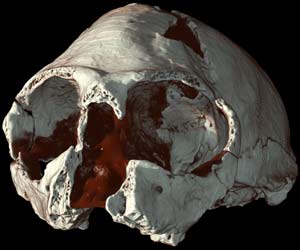
Literature
Baron, G., H. D. Frahm, K. P. Bhatnagar, and H. Stephan. 1983. Comparison of brain structure volumes in Insectivora and Primates. III. Main olfactory bulb (MOB). Journal für Hirnforschung 24:551-558.
Barton, R. A., A. Purvis, and P. H. Harvey. 1995. Evolutionary radiation of visual and olfactory brain systems in primates, bats and insectivores. Philosophical Transactions: Biological Sciences 348:381-392.
Cartmill, M. 1980. Morphology, function and evolution of the anthropoid postorbital septum; p 243-274 in R. Ciochon, B. Chiarelli (eds.), Evolutionary Biology of the New World Monkeys and Continental Drift. Plenum Press, New York.
Cave, A. J. E. 1973. The primate nasal fossa. Biological Journal of the Linnean Society 5:377-387.
Doving, K. B., and D. Troiter. 1998. Structure and function of the vomeronasal organ. Journal of Experimental Biology 201:2913-2925.
Firestein, S. 2001. How the olfactory system makes sense of scents. Nature
41:211-218.
Frahm, H. D., H. Stephan, and G. Baron. 1984. Comparison of brain structure volumes in Insectivora and Primates. V. Area striata. Journal für Hirnforschung 25:537-557.
Hershkovitz, P. 1974. A new genus of late Oligocene monkey (Cebidae, Platyrrhini) with notes on postorbital closure and platyrrhine evolution. Folia Primatologica 21:1-35.
Horovitz, I. 1999. A phylogenetic study of living and fossil platyrrhines. American Museum Novitates 3269:1-40.
Kay, R. F. 1990. The phyletic relationships of extant and fossil Pitheciinae (Platyrrhini, Anthropoidea). Journal of Human Evolution 19:175-208.
Kay, R. F., and E. C. Kirk. 2000. Ostological evidence for the evolution of activity pattern and visual acuity in primates. American Journal of Physical Anthropology 113:235-262.
Kay, R. F., V. M. Campbell, J. B. Rossie, M. W. Colbert, and T. B. Rowe. 2004. Olfactory fossa of Tremacebus harringtoni (Platyrrhini, Early Miocene, Sacanana, Argentina): implications for activity pattern. Anatomical Record, Part A, 281A:1157-1172.
Keverne, E. B. 1999. The vomeronasal organ. Science 286:716-720.
Kirk, E. C., and R. F. Kay. 2004. The evolution of high visual acuity in the Anthropoidea; p 539-602 in C. F. Ross and R. F. Kay (eds), Anthropoid Origins: New Visions. Kluwer/Plenum Publishing, New York.
Koppe, T., H. Nagai, and T. C. Rae. 1999. Factors in the evolution of the primate pneumatic cavities: possible roles of the paranasal sinuses; pp. 151-175 in T. Koppe, H. Nagi, and K. W. Alt (eds), The Paranasal Sinuses of Higher Primates. Quintessence Publ. Co. Inc., Chicago, Illinois.
Luchterhand, K., R. F. Kay, and R. H. Madden. 1986. Mohanamico hershkovitzi, gen et sp. nov., un primate du Miocène moyen d'Amérique du Sud. Comptes Rendus de l'Academie des Sciences, Paris 303(Ser. II):1753-1758.
Martin, R. D. 1973. Comparative anatomy and primate systematics. Symposium of the Zoological Society (London) 33:301-337.
Rossie, J. B. 2003. Ontogeny, homology, and phylogenetic significance of anthropoid paranasal sinuses. in Anthropology. Yale University, New Haven, CT.
Rusconi, C. 1933. Nuevos restos de monos fósiles del Terciario antiguo de la Patagonia. Anales de la Sociedad Científca Argentina 116:286-289.
Rusconi, C. 1935. Las especies de primates del oligoceno de Patagonia (gen. Homunculus). Revista Argentina de Paleontolia y Anthropolia 1:39-68 71-100.
Setoguchi, T., and A. L. Rosenberger. 1987. A fossil owl monkey from La Venta, Colombia. Nature 326:692-694.
Smith, T. D., M. I. Siegel, and K. P. Bhatnagar. 2001. Reappraisal of the vomeronasal system of catarrhine primates: ontogeny, morphology, functionality and persisting questions. Anatomical Record 265:176-192.
Stephan, H., H. D. Frahm, and G. Baron. 1981. New and revised data on volumes of brain structures in insectivores and primates. Folia Primatologica 35:1-29.
Stephan, H., H. D. Frahm, and G. Baron. 1984. Comparison of brain structure volumes in primates. VI: non-cortical visual structures. Journal für Hirnforschung 25:385-403.
Szalay, F. S., and E. Delson. 1979. Evolutionary History of the Primates. Academic Press, New York, 580 pp.
Van Valkenburgh, B., J. Theodor, A. Friscia, and T. Rowe. in press. Respiratory turbinates of Canids and Felids: A Quantitative Comparison. Journal of Morphology.
Wright, P. C. 1996. The neotropical primate adaptation to nocturnality: feeding in the night (Aotus nigriceps and A. azarae); pp. 369-382 in N. Norconk, A. L. Rosenberger, and P. Garber (eds), Adaptive Radiations of Neotropical Primates. Plenum Press, New York.




 ,
,